Reckeweg Award for the Best Clinical Case Report in Multitarget Therapies
Conditions of entry
You can now apply for the Reckeweg Clinical Case Award 2025.
The Deadline for eligible cases is Feb 28, 2025.
Background
By offering the Reckeweg Clinical Case Award, Heel GmbH supports healthcare professionals in publishing outstanding clinical work illustrating disease management with multitarget therapies, seeking to improve patient-centred care.
Reckeweg Award
Heel sponsors the €5,000 Reckeweg Award for the Best Clinical Case Report in Multitarget Therapies, which is given for excellent clinical case reporting that educates healthcare professionals about multitarget treatment options. The international review panel assesses all applications and selects winners based on the predefined criteria outlined below. In certain circumstances determined by the review panel, awards may also be shared.
Award Process
The application period is open for the whole year. The process is shown in the figure below.

Publication of clinical cases. Before applying for the Award, your case must be published in a medical journal or posted on the preprint platform (e.g. Figshare and OSF) not more than 3 years before the application date.
Application period. The deadline for application is February each year. After February, you can still apply for the next year’s Award.
Case review and winner selection. International Award Review Panel will review accepted cases, and up to three winners will be selected.
Ceremony Event. The winning authors (who filled out the application) will be invited to receive the official award certificate during a ceremony in Baden-Baden, Germany.
Award criteria
The €5,000 grant will be awarded for scientifically published or posted clinical cases that report on the use of multitarget therapies in everyday clinical practice. The Reckeweg Clinical Case Award is based on the following criteria:
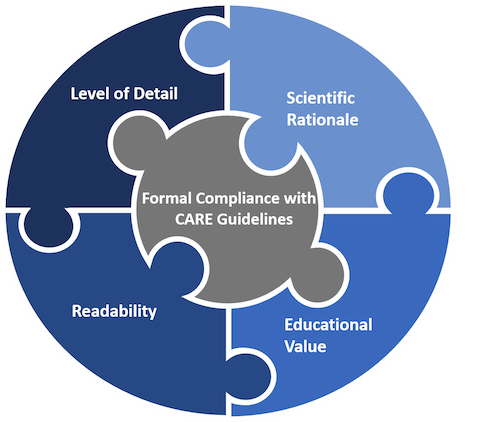
- Readability. The clinical case should be easy to read. Exhaustive explanations, very long sentences, and texts overloaded with professional jargon make case report tedious and exhausting to read. A good case report should have a balance between comprehensiveness of information and simplicity of the most critical points to understand for the reader.
- Formal compliance with the CARE guidelines. Authors should submit a completed CARE checklist to demonstrate that their report is written according to the CARE guidelines. The CARE checklist can be downloaded below.
- Level of detail. The patient narrative should provide sufficient detail for healthcare professionals to understand the course of events, diagnostic challenges, and dosage regimen in the case. Images or tables may be added to give more information to readers.
- Scientific rationale. In sufficient detail, the author(s) should explain the background to the report, the health issue addressed, the treatment options available, and why the case may be of interest. This information should be reflected in the introduction and discussion sections of the manuscript.
- Educational value for healthcare practitioners. The clinical case must explain how the information reported contributes to current medical knowledge. It should provide key points discussing the case’s generalisability and application in other settings.
Who should apply?
Application is open to all licensed healthcare professionals worldwide (human or veterinary). Employees of Heel GmbH and affiliated companies are not eligible to enter.
Application Procedure
Applicants should submit the following:
- A few sentences explaining why they submit the case.
- The working DOI hyperlink of their published or posted case report.
- A completed CARE checklist as a PDF.
- A signed authorship and copyright consent form.
Authors should complete the application via the application portal on this website.
Acceptance criteria
All applications will be checked for completeness before being accepted for evaluation by the international review panel. Check the application portal for more information.
To be considered for the review process, the application must:
- Be complete (DOI, authorship and copyright consent, and CARE checklist annotated with page numbers, not simply checks or crosses).
- Raise no ethical concerns (all patient data are anonymized, and there is a statement confirming that patients’ written informed consent has been obtained).
- Provide author contact information.
In a separate email, authors will be notified whether or not their application was accepted. Only official documents, which can be downloaded from the application portal, will be accepted. Applications containing old forms or materials other than the CARE checklist and authorship and copyright consent form will be automatically rejected.
Award review panel
The submitted applications will be assessed by an international panel of experts according to the award criteria. You will be notified about the decision in due time.


